No.10
20 November 2018

Hydrogels Absorbing moisture, releasing water
Takashi Miyata
Professor, Faculty of Chemistry, Materials and Bioengineering
(Osaka, 20 November 2018) Takashi Miyata at Kansai University and colleagues report in Nature Communications a temperature-responsive gel that absorbs moisture and, when heated, releases it in the form of water. Applications include energy-efficient materials for condensing moisture into water.
Hydrogels are highly absorbent materials, and when they incorporate polymer chains that respond to external stimuli (such as pH or temperature) they that can display abrupt volume changes when the surrounding conditions change. Such stimuli-responsive hydrogels have a range of potential applications, for example in drug delivery systems, sensors, cell culture and so on.
In particular, thermo-responsive hydrogels kept in an aqueous solution undergo a reversible phase transition above a critical temperature that causes a transition from a hydrated to a dehydrated state, which results in a drastic shrinking of the gel. Many studies exist on hydrogels that respond to changes in temperature while in solution, but the behavior of the dried gels in air has not been studied yet.
Now, Takashi Miyata at Kansai University and colleagues present in a study published in Nature Communications a temperature-responsive gel that absorbs moisture and, when heated, releases it in the form of water. The gel contains a thermo-responsive polymer and a hydrophilic component (sodium alginate) that boosts water absorption. The transition temperature for this material is close to room temperature, 32 ℃. When exposed to high relative humidity (80%) at 25℃, the gel absorbs 0.6 g of water per gram and swells. The presence of water on the polymer chains causes them to respond to temperature in a similar way as hydrogels immersed in solution, so that the chains, that are initially hydrophilic, change to hydrophobic with raising temperature. When this happens, the water molecules desorb and condense into liquid water. Indeed, as the sample is heated to a temperature of up to 50℃, water appears on its surface, and its amount increases sizably for temperatures above 40 ℃.
Thus the gel acts as a dehumidifier but, unlike traditional dehumidifiers that require energy to evaporate the absorbed water to regenerate the material, and then to condense it for collection, the gel can condense water simply in response to a small temperature change (from 25 ℃ to 50 ℃). Regeneration of the material and collection of the water can thus be achieved using very little energy.
Further optimization is still needed, but thermo-responsive gels like the one presented in this paper could find applications as energy-efficient materials for condensing moisture into water.
Background
Hydrogel
A hydrogel is a polymeric network formed by hydrophilic polymer chains; the hydrophilicity of the chains means that when immersed in waters hydrogels can absorb big quantities of water (a hydrogel can contain more than 90% water), which causes the gel to swell, maintaining its 3D structure. Hydrogels are used in many contexts, for example as scaffolds in tissue engineering, for sustained drug delivery or, if they contain additive sensitive to specific molecules, as biosensors.
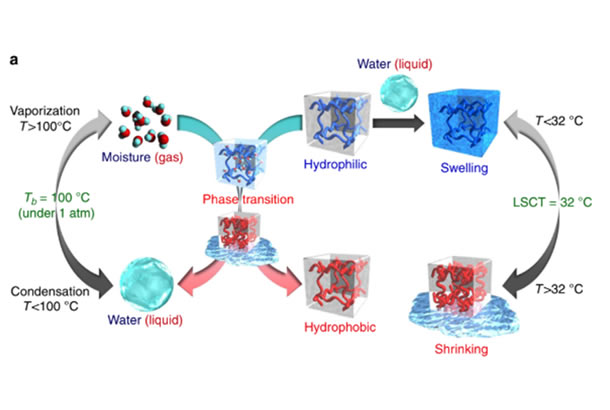
Caption
Illustration of the main concepts described in this paper.
Reference
Kazuya Matsumoto, Nobuki Sakikawa and Takashi Miyata, Thermo-responsive gels that absorb moisture and ooze water. Nat Commun. 9, 2315 (2018).
Further information
Kansai University, Japan
3-3-35 Yamate-cho,
Suita-shi, Osaka 564-8680 JAPAN
Website Kansai University
http://www.kansai-u.ac.jp/English/