No.3
21 March 2018

Novel adhesive and thermally stable epoxy resins
Hiroto Kudo
Professor, Faculty of Engineering Science
Epoxy resins or epoxies are organic compounds that can be hardened into adhesive materials with excellent thermal stability, mechanical strength, and chemical resistance. The hardening or 'curing' results from cross-linking either within the resin itself or between the resin and a co-reactant. Epoxies are produced by polymerization of epoxides — organic molecules with one or more three-atomic, triangluar carbon–oxygen–carbon (C–O–C) rings. Such rings exhibit a high strain, resulting in the easy polymerization of epoxies.
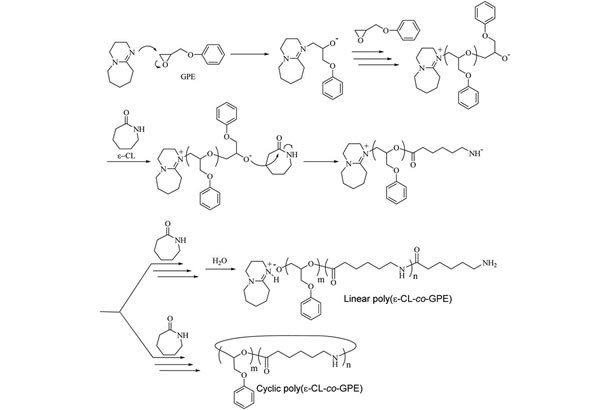
In order to develop cured epoxy resins with outstanding properties for use in industrial applications, it is crucial to fully understand the reaction mechanisms involved. With this in mind, Hiroto Kudo and Kentaro Buya at Kansai University, Osaka, Japan, looked at the reaction of ε-caprolactam (ε-CL) and glycidyl phenyl ether (GPE), both as a model system and as a promising approach towards the synthesis of novel cured resins. GPE is an epoxy, whereas ε-CL is a well-known co-reactant capable of polymerizing epoxies by opening C–O–C rings, also used in the synthesis of Nylon 6.
The researchers let ε-CL react with GPE in the presence of an organic compound abbreviated as DBU, acting as catalyst. After letting the mixture react for two hours at 170℃, they observed ring-opening copolymerization of the two reactants, with a poly(amide–ether) as the end product with a yield of 85%. A longer reaction time did not lead to a higher yield, nor did temperatures lower or higher than 170℃. The researchers also established the optimal concentrations of ε-CL, GPE and DBU and were able to propose a mechanism for the reactions taking place, suggesting that the poly(amide–ether) has a so-called pseudo-block gradient-copolymer structure.
Based on their findings on the ε-CL/GPE/DBU reaction mechanisms, the researchers developed other cured epoxy resins with ε-CL as the curing co-reagent. Their materials displayed adhesion, high glass-transition temperatures and excellent thermal stability, sufficient for commercialization.
Reference
Hiroto Kudo & Kentaro Buya. "Mechanistic Study of Ring-Opening Copolymerization of E-Caprolactam with Epoxide: Development of Novel Thermosetting Epoxy Resin System." Journal of Polymer Science, Part A: Polymer Chemistry, 54, 2220–2228, (2016).
DOI: 10.1002/pola.28095
http://onlinelibrary.wiley.com/doi/10.1002/pola.28095/abstract